This morning I released a new version of my eBook “Clinical Variant Analysis for Cancer – Second Edition.” The clinical utilization of Next-Generation Sequencing data to diagnose cancer has taken off, resulting in the need to standardize the interpretation and reporting of observed genomic variations. This eBook explores the entire clinical diagnostic process. It demonstrates how Golden Helix software can support clinicians with their… Read more »
Thank you to everyone who joined me for our latest webcast, “Next-Gen Sequencing of the SARS-CoV-2 Virus with Golden Helix.” If you missed the live event and are interested in knowing what we talked about, you may access the recorded event below: Our Live Q&A generated a lot of great questions. Unfortunately, we were unable to answer them all, but… Read more »
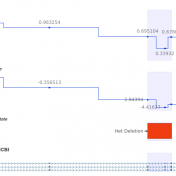
In trio workflows, one of the most important factors in scoring a variant is understanding how that variant is inherited from the parents. Likewise, when looking at extended families, the segregation, or presence of the variant among the affected versus unaffected individuals provides evidence for its pathogenicity for a given phenotype or disease. Given the nature of Copy Number Variants… Read more »
VarSeq 2.2.1 was released on April 1st and features an upgraded gene annotation capability with new RefSeq genes tracks and an AMP workflow addition: the Drugs and Trials tab. The new RefSeq human genome genes tracks contain updated gene names and the recognition of any MANE (Matched Annotation from NCBI and EMBL-EBI) identified transcripts. VarSeq has been updated to be… Read more »
Thank you to everyone who joined me for yesterday’s webcast, I hope you all enjoyed it. If you missed the live event and are interested in knowing what we talked about, good news, you can watch the recorded version right here! There were so many great questions asked during our Live Q&A that I was unable to answer all of… Read more »
Our Support Team curates a variety of tutorials to help orient new users to the capabilities of VarSeq. We are happy to announce the team’s new release of the trio tutorial that places emphasis on using the ACMG guidelines. This tutorial gives insight into the proper setup of pedigree structure as well as detailed descriptions of the filter containers and… Read more »
In these very uncertain times, it has been uplifting to read the wonderful work Golden Helix customers have been doing across the globe! This month, the focus of this blog is on published articles relating to congenital defects and early development. Congratulations to all our customers who have published papers and please enjoy a small sampling of their work. To… Read more »
Generating a clinical report is the final step of most NGS pipelines and is important as it relays results and information to legacy systems, physicians and ultimately the patient. As reporting is a valuable process, Golden Helix offers reporting capabilities according to the ACMG and AMP guidelines but also as a standalone feature in VSReports. VSReports is a platform that… Read more »
At Golden Helix, we want our new users to hit the ground running with VarSeq and not spend oodles of time getting started building and automating their workflows. To achieve this goal, our team has generated blogs, webcasts, and tutorials that explain and demonstrate workflows that are possible with VarSeq. Each VarSeq tutorial offers step by step instruction in which… Read more »
Since the initial release of the copy number variant algorithms in VarSeq, our team has created a variety of content to help users get started with building their copy number variant projects. In our webcast library, you can find a few of our recent webcasts in 2019 covering CNV workflows and validation: Or, if you prefer to take a more… Read more »
Golden Helix is in a unique position to provide a secure on-premise analysis solution. This capability is based on two enablers. First, we build our software solutions from scratch and from the ground-up with the assumption that it should run on any operating system and potentially behind firewalls or even without internet access. Second, we provide these solutions on a licensing model based on training and supporting users, not… Read more »
As many of our users know, VarSeq comes shipped with various project templates that are designed to give users a baseline workflow to get started with their projects. These templates are tailored for various applications including tumor-normal, trios, cancer and hereditary gene panels, and ACMG Guidelines workflows. The templates contain application-specific annotation sources and algorithms that will automatically load into… Read more »
Customizing VSClinical ACMG Guidelines Workflow Part 2 In the first part of this series, we covered how VarSeq provides customization of the clinical analysis workflow process. VSClinical’s various customization parameters within the ACMG Guidelines workflow includes the choice of how the internal knowledge base of previous variant interpretations are stored and what considerations go into this choice. In this blog, we… Read more »
Hospitals and testing labs are undergoing a digital transformation like any other business in our time. They deal with personal data in categories with the highest level of security requirements: personal identity and medical records. The architecture chosen to store and analyze this data is critical to provide the best protection against a data breach incident. The liability and institutional… Read more »
When interpreting a variant using the AMP/ASCO guidelines for somatic variant interpretation, clinicians must determine whether the variant can be considered a biomarker that affects clinical care by predicting sensitivity, resistance, or toxicity to a specific therapy. Such a determination requires the investigation of multiple evidence sources, including clinical trials, FDA approved therapies and peer-reviewed studies. Unfortunately, strong evidence linking… Read more »
Clinical labs offer a unique and sophisticated product that is performed repeatedly with high standards of quality. VarSeq was developed to provide labs with the customization required for clinical genetic tests in a repeatable workflow. On top of this, VSClinical offers additional parameters and choices that can be made when designing the test workflow. In this blog series, we will… Read more »
Genetic testing labs deal with personal data in categories with the highest level of security requirements: personal identity and medical records. Given the liability and risk associated with a breach of this secure information, it is not surprising that many labs and institutes that aggregate genomic data prefer, if not require, on-premise analysis and storage solutions. Golden Helix is in… Read more »
As clinical genetic tests have been adopted as a critical enabler of precision medicine, the number of tests offered by clinical labs and the volume of tested patients has grown by orders of magnitude in the past five years. The Gene Testing Registry, managed by the NIH, documented a rise from 13,000 to 60,000 tests offered in the US market… Read more »
The first two blogs in this series covered Sentieon’s somatic variant calling from Tumor with Normal (Part I) and Tumor without Normal (Part II). In addition to providing multiple somatic variant calling processes, users also have access to high-sensitivity scripts and full support for the GRCh38 reference assembly. Without going into excessive detail, this final blog of the series will… Read more »
Our team has returned from the annual meeting of the Association of Molecular Pathology (AMP 2019), and, as always, I am grateful for all the wonderful experiences we are bringing back with us. The plenary sessions and talks were bountiful, and we were very impressed with the well-organized exhibition. Hats off to everyone involved in planning this great event! Innovation… Read more »