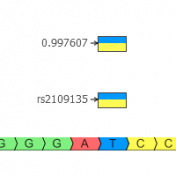
We are happy to announce that our latest version of SVS includes the ability to call CNVs on low read depth Whole Genome Sequencing (WGS) data. Designed for calling large cytogenetic events, this algorithm can detect chromosomal aneuploidy events and other large events spanning one or more bands of a chromosome from genomes with average coverage as low as 0.05x…. Read more »
This blog will conclude our VSClinical Best Practice Workflow series and focuses on one of our new reports: VSClinical ACMG Gene Panel Template. This template is valuable because it automatically enters your variant interpretation from the ACMG Guidelines into the report and eliminates the need for manual submission. I would like to explain how to properly implement this report into… Read more »
In part one of this series, we discussed how the ACMG Classifier can be implemented in your filter chain to support a best practice workflow. To continue our discussion on best practices of VSClinical, this blog will shed light on other attributes of VSClinical that can add support to your evaluation. Specifically, we will explore how VSClinical can help users… Read more »
In the first two parts of this blog, we presented examples of how to leverage Warehouse-stored VSClinical and CNV assessment catalogs in the VarSeq project. Now we are going to explore the Warehouse interface a bit more and show how to query on stored variant data. To access Warehouse from VarSeq, click the V Connect icon located in the top… Read more »
Part 1 of this blog series was focused on new capabilities in Warehouse to store your CNV results. We explored some approaches of how to utilize assessment catalogs of cohort and known pathogenic events. What makes Warehouse so useful in this application is that the catalog is accessed from one central location and ensures every user is leveraging the same… Read more »
We recently hosted a webcast covering the value and application of VSWarehouse through VarSeq. Not only is VSWarehouse a solution for storing your NGS data in a central repository, but it also provides a means to enhance the tertiary analysis done in VarSeq. VSWarehouse will store all your sample/variant data but also stores your catalogs of pathogenic variants, clinical reports, and has the capability of filtering/querying on all your stored data quickly. In addition,… Read more »
This webcast generated some great questions! If you have any other questions for me that are not answered below, please feel free to ask those by emailing [email protected]. To what level does the Warehouse scale? We have tested multiple instances of Warehouse in-house and on the cloud and it scales incredibly well to tens of thousands of samples and 100s… Read more »
With this two-part blog series, users should now be able to perform CNV analysis using their data, set up basic quality filter standards to isolate high-quality events and utilize annotations to hone in on publicly known events as well as in-house recorded CNVs from previous projects.
The recent release of VSClinical gives users the ability to evaluate variants based on the 33 criteria according to the American College of Medical Genetic and Genomics (ACMG) guidelines. This feature leverages a variety of variant sequencing evidence including population data, functional data, and computational predictions while providing rich visualizations and auto recommendations to help answer challenging criteria. This highly… Read more »
Ready to take your analysis to the next level? Our Small-Lab VarSeq PowerPack enables users to move their analysis from FASTQ to clinical reporting through one streamlined pipeline. Here’s what’s included: VarSeq VarSeq is an intuitive, integrated software solution for tertiary analysis. With VarSeq you can automate your workflows and analyze variants for gene panels, exomes, and whole genomes. VS-CNV… Read more »
Relating human phenotypes to genotypes is the name of the game with OMIM, and as their website says, “is intended for use primarily by physicians and other professionals concerned with genetic disorders, by genetics researchers, and by advanced students in science and medicine.” The Online Mendelian Inheritance in Man (or OMIM) was originally created by Dr. Victor A. McKusick in… Read more »
There are many good reasons why the pursuit of the highest quality genomic interpretation would lead you to the latest human reference. It is more complete and fixes incorrect or partially missing genes that have known implications for human disease. While most major projects cataloging human populations have plans to re-do all their genomic alignments to the new human reference… Read more »
2017 was an incredibly prosperous year for Golden Helix; we released a handful of new features, announced new partnerships and completed our end-to-end architecture for clinical testing labs. Our webcast series has become a very popular way for our community to stay up-to-date with our new capabilities and best practices in genetic analysis using our software. We had three webcast… Read more »
We have a lot to thank the 1000 Genomes project for in the genomics community. By the collaborate efforts of many researchers and organizations, the project produced not only the first catalog of rare human variation but in the process standardized many things we take for granted, such as the VCF and BAM file formats. The variant frequencies of the… Read more »
Golden Helix is excited to announce a new round of novel and updated annotations; including a frequency track, a region track, and a gene track. All three of these tracks were created with the use of VarSeq and its Convert Wizard functionality. First, the expansive 1000 genomes track (1kG) has been updated to include sub-population allele frequencies and heterozygous and… Read more »
With the recent release of VarSeq 1.4.7, we have expanded the concepts of our popular assessment catalog to include CNV and other region-based records and not just variants. To match these capabilities, we have made a major update to VSWarehouse that supports these new record types in the centrally hosted and versioned Catalogs and Reports. Review of the VSWarehouse Genomic… Read more »
In our final chapter of this variant annotation blog series, we will discuss additional annotations that provide powerful variant filtering and analysis capability. Golden Helix curates many annotations in a way that allows for simple analysis and saves the users the hassle of all this data management. Whether you are trying to capture rare variants known across multiple subpopulations in… Read more »
This month we hosted two, incredible webcasts officially announcing the latest CNV annotation capabilities our Software Engineering Team has been hard at work on for the past couple of months. Our first webcast, Comprehensive Clinical Workflows for Copy Number Variants in VarSeq, was presented by Golden Helix’s VP of Product & Engineering, Gabe Rudy, who reviewed the expanded capabilities of… Read more »
CIViC The Clinical Interpretations of Variants in Cancer, better known as CIViC, is an open access open source, community-driven web resource available to all VarSeq users. Nature Genetics published an article that states, “CIViC accepts public knowledge contributions but requires that experts review these submissions”. Fundamentally, the focus behind CIViC is to make sure the variants contained in the database… Read more »
Gabe Rudy gave a fantastic presentation yesterday on the latest additions to VS-CNV annotations. If you weren’t able to join us for the live event, you can access the recording and webcast slides here: Comprehensive Clinical Workflows for Copy Number Variants in VarSeq. Additionally, there were many great questions asked that we wanted to share with the community. Question: Can I… Read more »