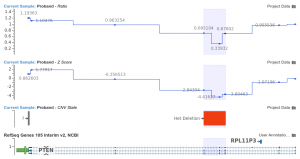
Auto-Updating Template Sources

In VarSeq 2.2.1, you can enable auto-updating for template annotation sources, ensuring they always reflect the latest available version. Previously, VarSeq templates were frozen in time. Now, each new project created from a template would use the same source that was used when the template was created. When you save…
Read more →