Featured in The Journal of Precision Medicine: Implementing the ACMG Guidelines for CNV in a Commercial Software Solution

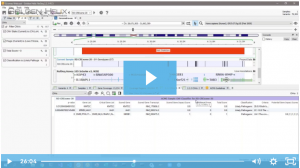
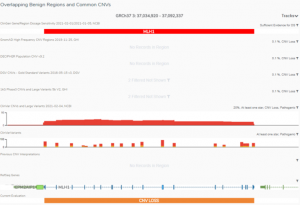
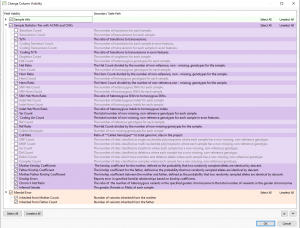
We are excited to share our latest publication with The Journal of Precision Medicine, “Implementing the ACMG Guidelines for CNV in a Commercial Software Solution”. “In 2020, ACMG in collaboration with the ClinGen working group developed a new set of guidelines for the clinical interpretation of CNVs. While theseguidelines provide…
Read more →