SVS offers several options to conduct genome wide association tests and mixed linear models. At times, it can be challenging to decide which test, model, or adjustments to use when setting up your analysis. I want to briefly explore the options available in SVS for association tests, and mixed linear models to hopefully facilitate in understanding and choosing which options… Read more »
This morning I released a new version of my eBook “Clinical Variant Analysis for Cancer – Second Edition.” The clinical utilization of Next-Generation Sequencing data to diagnose cancer has taken off, resulting in the need to standardize the interpretation and reporting of observed genomic variations. This eBook explores the entire clinical diagnostic process. It demonstrates how Golden Helix software can support clinicians with their… Read more »
Thank you to everyone who joined me for our latest webcast, “Next-Gen Sequencing of the SARS-CoV-2 Virus with Golden Helix.” If you missed the live event and are interested in knowing what we talked about, you may access the recorded event below: Our Live Q&A generated a lot of great questions. Unfortunately, we were unable to answer them all, but… Read more »
It is an honor to be featured by Clinical Lab Manager where I discuss how Next-Gen Sequencing can be leveraged in the fight against COVID-19. You can access this article directly by visiting https://www.clinicallabmanager.com/thought-leadership/leveraging-next-generation-sequencing-technology-in-the-fight-against-covid-19-22584. If this is an area of interest for your organization, please reach out to our team and we would be happy to discuss this in more detail.
Scientific investigations and genetic discoveries continue to happen in several diverse areas even while our world is currently consumed by the greatest health challenge of our era. Below are a few examples of some of the great work being done in the fields of human medicine, ecology, and breed conservation of animal species. As always, we are so very proud… Read more »
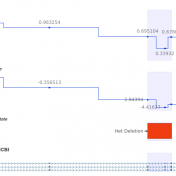
In trio workflows, one of the most important factors in scoring a variant is understanding how that variant is inherited from the parents. Likewise, when looking at extended families, the segregation, or presence of the variant among the affected versus unaffected individuals provides evidence for its pathogenicity for a given phenotype or disease. Given the nature of Copy Number Variants… Read more »
We are honored to be recognized in this year’s 2020 Biotechnology Awards by GHP Magazine – thank you! Find the full list of winners here. “Covering a variety of verticals, including bio agriculture, bio services, bio industrial and biopharmaceutical, the biotechnology market are under the microscope in Global Health & Pharma’s Biotechnology Awards. Boasting impressive market value growth with no… Read more »
VarSeq 2.2.1 was released on April 1st and features an upgraded gene annotation capability with new RefSeq genes tracks and an AMP workflow addition: the Drugs and Trials tab. The new RefSeq human genome genes tracks contain updated gene names and the recognition of any MANE (Matched Annotation from NCBI and EMBL-EBI) identified transcripts. VarSeq has been updated to be… Read more »
Thank you to everyone who joined me for yesterday’s webcast, I hope you all enjoyed it. If you missed the live event and are interested in knowing what we talked about, good news, you can watch the recorded version right here! There were so many great questions asked during our Live Q&A that I was unable to answer all of… Read more »
For more than 20 years, Golden Helix has focused on our mission to empower precision medicine around the world. We want you to know you have our commitment to continue providing you with the products and services you depend on during these unprecedented times. Today, we have announced our latest efforts to help assist the clinical research community in this… Read more »
Our Support Team curates a variety of tutorials to help orient new users to the capabilities of VarSeq. We are happy to announce the team’s new release of the trio tutorial that places emphasis on using the ACMG guidelines. This tutorial gives insight into the proper setup of pedigree structure as well as detailed descriptions of the filter containers and… Read more »
In these very uncertain times, it has been uplifting to read the wonderful work Golden Helix customers have been doing across the globe! This month, the focus of this blog is on published articles relating to congenital defects and early development. Congratulations to all our customers who have published papers and please enjoy a small sampling of their work. To… Read more »
At Golden Helix, customer support is our number one priority. This support also includes the health and safety of all the members of our community. During this time of uncertainty, we first want to acknowledge the incredible efforts of the healthcare industry around the globe, working so hard to conquer this outbreak. Secondly, we realize a significant portion of our… Read more »
Today, CIO Look announced their collection of the Top 10 World’s Most Trust Worthy Bioanalysis Companies in 2020. We are incredibly honored to have received this award and being recognized amongst the top biotech companies! You can access the publication here: https://ciolook.com/golden-helix-inc-empowering-healthcare-organizations-with-industry-leading-next-generation-sequencing-analysis-software/. As always, we would not be receiving this recognition without the incredible support from our community. Thank you… Read more »
Generating a clinical report is the final step of most NGS pipelines and is important as it relays results and information to legacy systems, physicians and ultimately the patient. As reporting is a valuable process, Golden Helix offers reporting capabilities according to the ACMG and AMP guidelines but also as a standalone feature in VSReports. VSReports is a platform that… Read more »
Introducing Drugs & Trials for Cancer Diagnostics VSClinical offers enormous simplicity and consistency in evaluating biomarkers and providing treatment options. Last month, we announced our newest feature to now include the automated collection of relevant clinical trials. The new capability was unveiled in our “Introducing Drugs & Trials for Cancer Diagnostics” webcast with Nathan Fortier, Ph.D., Director of Research, showing… Read more »
At Golden Helix, we want our new users to hit the ground running with VarSeq and not spend oodles of time getting started building and automating their workflows. To achieve this goal, our team has generated blogs, webcasts, and tutorials that explain and demonstrate workflows that are possible with VarSeq. Each VarSeq tutorial offers step by step instruction in which… Read more »
Our customers are making great contributions to human research and precision medicine every month! In February, VarSeq’s Clinical Suite dominated the published works citing Golden Helix. It is an honor for us to be at the forefront of so many important genetic investigations and we appreciate the efforts of those who work for the greater good. Read further to see… Read more »
Although VarSeq is intentionally designed to be a clinical NGS pipeline tool able to run a handful or even single samples through, we have many users who run large cohort style studies with the tool as well. One common use is to compare case/control data to isolate variants shared among affected individuals and exclude those in unaffected. One incredibly powerful… Read more »
Since the initial release of the copy number variant algorithms in VarSeq, our team has created a variety of content to help users get started with building their copy number variant projects. In our webcast library, you can find a few of our recent webcasts in 2019 covering CNV workflows and validation: Clinical Validation of Copy Number Variants Using the… Read more »