With the release of VarSeq 2.2.4 just around the corner, I want to detail some new 2.2.4 features that will enhance somatic variant annotation and fusion analysis within VSClinical. A webcast back in July showed some of these updates in action, so if you are looking for some more content on this topic, I would highly recommend checking out the… Read more »
Golden Helix VSClinical provides a guided workflow interface for following the ACMG and AMP guidelines to evaluate variants and CNVs for NGS tests. The output of this work is most often a lab-specific clinical report. Since it was introduced, we have provided a powerful Word-based templating system to allow labs the ability to generate customized reports to include specific content… Read more »
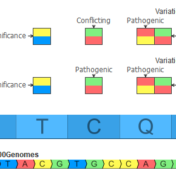
In the September 2021 monthly update to our curated ClinVar track, we made some changes that will result in roughly another 7,000 Likely Pathogenic and Pathogenic variants being available for annotation and use in the ACMG auto-classification system. Consensus Between Labs ClinVar has nearly one million unique variant classification records that are curated into multiple annotation tracks used in VarSeq and VSClinical on a monthly basis. Clinical… Read more »
Established in 2004 and headquartered in Chennai, India, with regional centers across the country, LifeCell runs India’s largest stem cell bank and has also diversified into diagnostics and tissue therapeutics. They employ roughly 2,000 people, providing genetic services to customers in the mother and baby space. Phani Nagaraja Setty is working as a scientist at LifeCell. Setty obtained his Master’s… Read more »
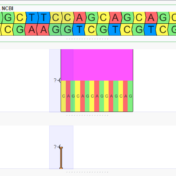
Merging variant records, VCFs, across samples is important when performing trio or family analysis as it ensures that hereditary relationships can be properly inferred. There are many ways to represent a single variant. Insertions and deletions may be right or left aligned, prefixes and suffixes can be added, and adjacent variants in the same sample may be combined or split… Read more »
We would like to thank everyone who participated in our 2021 t-shirt design competition. It was great to see the amount of creativity our community expressed and was certainly a tough decision to make! We are pleased to announce this year’s winners: First Place – Bruce Eng For over 35 years, Bruce Eng has partnered with businesses, educational institutions, and… Read more »
Welcome to the August 2021 edition of our customer publications blog post! Each month we spotlight a few recently published articles by our incredible Golden Helix customers. With users spanning both research and clinical spaces, the topics vary widely across many fields. This month, we will be highlighting VSClinical users and the guided workflow. Host Genetics and Antiviral Immune Responses… Read more »
Join the Golden Helix team at this year’s ESHG 2021 Virtual Conference! We will be presenting two different talks on our different product solutions and fielding any questions you might have. VSClinical: a comprehensive NGS clinical solution The first talk, VSClinical: a comprehensive NGS clinical solution, will be on Sun, 29 August, 14:00-15:00. This will be moderated by Golden Helix… Read more »
Clinical diagnostic efforts in next-generation sequencing are commonly defined at a gene panel level. The validation process of adding new genes to any diagnostic panel is ongoing, but labs typically construct and validate their clinical workflows for the current status of verified genes. This is not limited to primary finding results but can also include any incidental findings among the… Read more »
Clinical labs often maintain gene panels, which are lists of genes with evidence of disease association. These panels are used to prioritize variants and limit interpretations to a predefined set of test-specific genes. In general, gene panels should be stored independently of any specific project or interpretation, as it is common for an individual gene panel to be generally applicable… Read more »
Inc. magazine today revealed that Golden Helix, Inc. is No. 3267 on its annual Inc. 5000 list, the most prestigious ranking of the nation’s fastest-growing private companies. The list represents a unique look at the most successful companies within the American economy’s most dynamic segment—its independent small businesses. Intuit, Zappos, Under Armour, Microsoft, Patagonia, and many other well-known names gained… Read more »
I remember visiting a patient in the NICU amongst the incubators, some glowing blue like tiny tanning beds to treat jaundice, all containing tiny humans – many smaller than a loaf of bread. Infants get admitted into the neonatal intensive care unit or NICU for many reasons ranging from elevated bilirubin, hypoglycemia, sepsis, and respiratory distress (RDS). Many are eventually… Read more »
In the July 2021 Customer Publication blog, I will highlight four studies that provided further insights into conditions that are typically identified in early childhood. As you will see as you read the summaries of each publication, both Golden Helix software platforms (VarSeq and SNP & Variation Suite or SVS for short) were instrumental in exploring the genetic factors that… Read more »
This blog post will cover an exciting new VSClinical feature in the upcoming VarSeq release. The ACMG Previously Interpreted Variants feature allows users to integrate databases of expert-curated variant interpretations into their VSClinical workflows. These data sources store variant-level interpretation data, including the classification, associated disorders, interpretation text, and scored criteria for each variant, along with notes providing a justification… Read more »
We had a wonderful turnout for our recent webcast, “Reduce Turn-Around with Enhanced Cancer Annotations & Golden Helix CancerKB Updates.” Thanks so much to those who were able to join us! And not to worry, we have a link to the recording here just in case you weren’t able to make it. We covered the gamut of new and updated annotation tracks available in VarSeq along… Read more »
Golden Helix is proud to be recognized as a top biotechnology solution provider, named one of MirrorReview’s 10 Innovative Biotechnology Solution Companies for 2021. This acknowledgment reflects over two decades of dedication to advancing bioinformatics and empowering the global genomics community. Our success would not be possible without the support of our incredible customers, partners, and team members. If you’re… Read more »
The Genome Aggregation Database (gnomAD) is a resource developed by an international coalition of investigators, with the goal of aggregating and harmonizing both exome and genome sequencing data from a wide variety of large-scale sequencing projects (1). We have covered this annotation in-depth in other blog posts, but this resource contains over 125,000 exome sequences and around 16,000 whole genome… Read more »
Could leveraging CNV analysis for whole exome or genome sequencing help provide answers for the undiagnosed? Roughly 4% of the world’s population shares one common desire – a diagnosis (1). Though it may seem counterintuitive, a diagnosis, no matter how grim, can provide relief, validation, the chance to make plans, and have a discernible sense of future for individuals and… Read more »
Golden Helix’s solutions are leveraged in a wide variety of scientific fields. As always, we are thrilled to showcase some of the newly published articles written by our customers in June 2021. Here are a few that utilized VarSeq’s powerful scalability analyzing whole exome sequencing data. WES can identify variations in the protein-coding region of any gene. Since most known… Read more »
One of the many tricks of encoding so much functionality into so little space in eukaryotic genomes is the ability to produce multiple distinct mRNAs (transcripts) from a single gene. While one transcript is often the dominant one for a given tissue or cell type, there are, of course, exceptions in the messy reality of biology. It doesn’t take many… Read more »