It is an honor to be published in the European Journal of Clinical and Biomedical Sciences (Volume 6, Issue 4) in an article co-authored by Dr. Christiane Scherer and myself, “Investigating the Global Spread of SARS-CoV-2 Leveraging Next-Gen Sequencing and Principal Component Analysis.” This study highlights how genomic surveillance of COVID-19 using Next-Generation Sequencing (NGS) can uncover transmission patterns and mutation… Read more »
As I prepared to write the Customer Publication blog for June 2020, I was excited by the number of recently published papers that stood as examples of how both VarSeq and SVS software are employed to advance diagnostics and treatments in human medicine. We often think of SVS as the go-to platform for Agrigenomics, however both of our platforms have… Read more »
It is an honor to be featured in the Clinical OMICs May/June 2020 issue in a Q&A with the Editor discussing the past, present, and future of Golden Helix. In this article, I detail: Clinical OMICs Article: What has been Golden Helix’s most significant success or contribution to the industry over the past five years? Golden Helix started in 1998 with a… Read more »
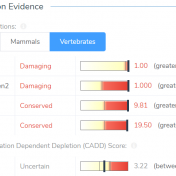
The University of Washington’s Combined Annotation Dependent Depletion (CADD) algorithm, now available in CADD v1.4 and CADD v1.5, measures the deleteriousness of genetic variants. This includes single nucleotide polymorphisms (SNVs) and short insertions and deletions (indels) throughout the human reference genome assembly. This algorithm was introduced in 2014 and has since become one of the most widely used tools to assess human… Read more »
Abstract Before assessing the clinical significance of a somatic mutation, one must determine if the mutation is likely to be a driver mutation (i.e. a mutation that provides a selective growth advantage, thereby promoting cancer development). To aid clinicians in this process, VSClinical provides an oncogenicity scoring system, which uses a variety of metrics to classify a given somatic mutation… Read more »
An under-appreciated area of complexity when looking into the field of genetics from the outside can be found in genes and transcripts. Alternative splicing allows eukaryotic species to have a wonderfully powerful genetic code, resulting in multiple protein isoforms being encoded in a single section of DNA. But when it comes to variant interpretation, different transcripts can result in widely different predicted… Read more »
Writing the blog post to summarize and highlight our customer’s publications is undoubtedly one of my favorite things to do! The wide variety of topics is always surprising and inspiring, and I am humbled by the efforts of dedicated scientists who are helping to protect and enrich our lives in so many ways. Our SNP & Variation Suite (SVS) software… Read more »
At Golden Helix, we’re committed to advancing precision oncology through robust software solutions that streamline clinical workflows. As the field of cancer genomics continues to evolve, staying informed about the latest best practices and tools is more important than ever. That’s why we’re excited to announce the release the second edition of Clinical Variant Analysis for Cancer eBook from our… Read more »
Thank you to everyone who joined me for our latest webcast, “Next-Gen Sequencing of the SARS-CoV-2 Virus with Golden Helix.” If you missed the live event and are interested in knowing what we talked about, you may access the recorded event below: Our Live Q&A generated a lot of great questions. Unfortunately, we were unable to answer them all, but… Read more »
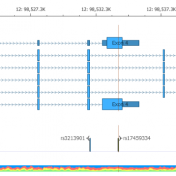
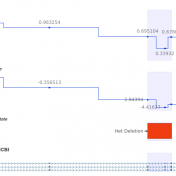
In trio workflows, one of the most important factors in scoring a variant is understanding how that variant is inherited from the parents. Likewise, when looking at extended families, the segregation, or presence of the variant among the affected versus unaffected individuals provides evidence for its pathogenicity for a given phenotype or disease. Given the nature of Copy Number Variants… Read more »
VarSeq 2.2.1 was released on April 1st and features an upgraded gene annotation capability with new RefSeq genes tracks and an AMP workflow addition: the Drugs and Trials tab. The new RefSeq human genome genes tracks contain updated gene names and the recognition of any MANE (Matched Annotation from NCBI and EMBL-EBI) identified transcripts. VarSeq has been updated to be… Read more »
Thank you to everyone who joined me for yesterday’s webcast, Using VarSeq Templates to Advance and Customize Variant Analysis, I hope you all enjoyed it. If you missed the live event and are interested in knowing what we talked about, good news, you can watch the recorded version right here! There were so many great questions asked during our Live… Read more »
Our Support Team curates a variety of tutorials to help orient new users to the capabilities of VarSeq. We are happy to announce the team’s new release of the trio tutorial that places emphasis on using the ACMG guidelines. This tutorial gives insight into the proper setup of pedigree structure as well as detailed descriptions of the filter containers and… Read more »
In these very uncertain times, it has been uplifting to read the wonderful work Golden Helix customers have been doing across the globe! In March 2020, the focus of this blog is on published articles relating to congenital defects and early development. Congratulations to all our customers who have published papers and please enjoy a small sampling of their work…. Read more »
We’re thrilled to share that Golden Helix has been recognized by CIO Look as one of the Top 10 Most Trustworthy Bioanalysis Companies in the World. This recognition highlights our dedication to supporting clinical labs and healthcare organizations with cutting-edge, affordable, and reliable software solutions for genomic data analysis. For more than two decades, Golden Helix has remained focused on… Read more »
Introducing Drugs & Trials for Cancer Diagnostics VSClinical offers enormous simplicity and consistency in evaluating biomarkers and providing treatment options. Last month, we announced our newest feature to now include the automated collection of relevant clinical trials. The new capability was unveiled in our “Introducing Drugs & Trials for Cancer Diagnostics” webcast with Nathan Fortier, Ph.D., Director of Research, showing… Read more »
Our customers are making great contributions to human research and precision medicine every month! In February 2020, VarSeq’s Clinical Suite dominated the published works citing Golden Helix. It is an honor for us to be at the forefront of so many important genetic investigations and we appreciate the efforts of those who work for the greater good. Read further to… Read more »
Golden Helix is in a unique position to provide a secure on-premise analysis solution. This capability is based on two enablers. First, we build our software solutions from scratch and from the ground-up with the assumption that it should run on any operating system and potentially behind firewalls or even without internet access. Second, we provide these solutions on a licensing model based on training and supporting users, not… Read more »
We are incredibly grateful to be recognized as one of the Top 60 Genetics Blogs on the Web by Feedspot. Our team is dedicated to educating our readers on how our solutions can help enable precision medicine, and we are so honored to have received this recognition. On our blog, you will discover posts touching on important topics, like cyber security strategies,… Read more »
Data and cyber security breaches are a fact of life. Organizations are aware of the threats and putting their best efforts up to prevent them as much as possible. Industries across the entire spectrum have been exposed in recent years. I will go through a few examples to show how pervasive this issue has become. Target One prominent data breach occurred… Read more »