VarSeq 2.3.0 unleashed a whole new way to select, process, analyze, and report cancer variants through complete workflow automation, application of evaluation scripts, and enhanced annotation. Single nucleotide variants, copy number variants, structural variants, and genomic signatures could be added to a single patient evaluation, and Golden Helix CancerKB came packed with new report-ready interpretations to support them!
Soon after, VarSeq 2.4.0 was released, which expanded the interpretation capabilities of VSClinical AMP to improve the capture of tier II variants. Between these two releases, there is a lot to process, and everyone wants to make sure they have their VarSeq workflow optimized and locked down to include all of the variants and analytical information they need to help provide the best treatment recommendations for cancer patients.
Thus I am here to help provide insight and guidance on how you can set up your VSClinical AMP workflow to capture the interpretations you need! In particular, we will focus on optimizing which tier II interpretations are reviewed and reported. The key to setting up the best tier II capturing workflows in VSClinical AMP will require us talking A LOT about evaluation scripts and evaluation options so if you are not familiar with using these yet, it’s time to jump in!
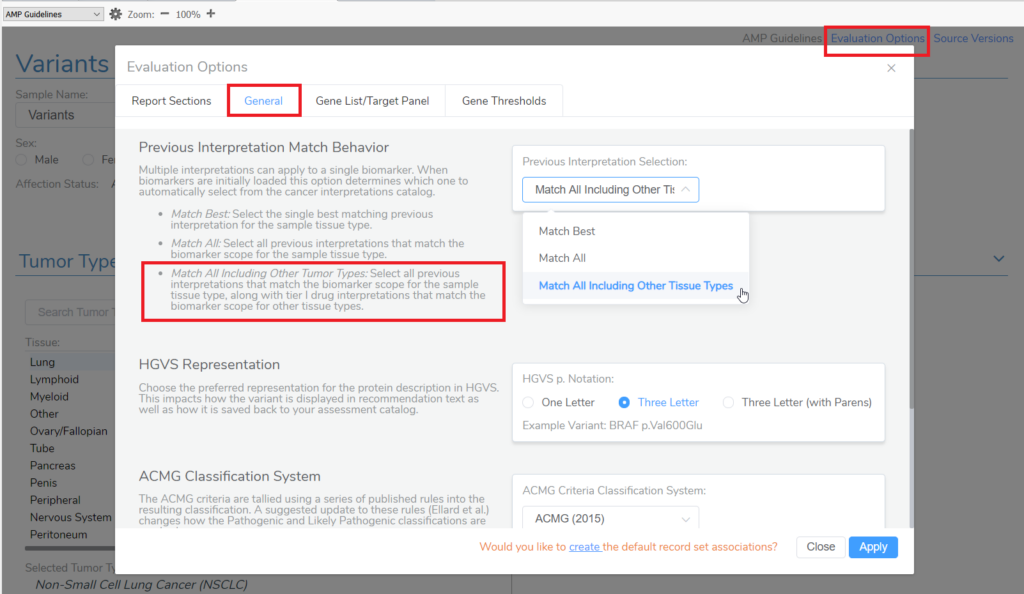
Crack open VSClinical, select the AMP workflow, and then STOP! Before even creating an evaluation, this is the first opportunity to start customizing which interpretations will apply to the variants that will get added to the patient evaluation via the Evaluation Options. Namely, by manipulating this setting, you can grab the tier II interpretations that meet the AMP Guidelines for a drug approved for treatment in another tumor type. Under the General tab, you have the option to choose how interpretations from both your internal assessment catalog of saved interpretations and how interpretations within Golden Helix CancerKB will match the variants added to evaluations that are created within the project. Selecting the third option, “Match All Including Other Tumor Types,” will automatically add interpretations to an evaluation for a biomarker that has approved drug treatments in other tumor types.
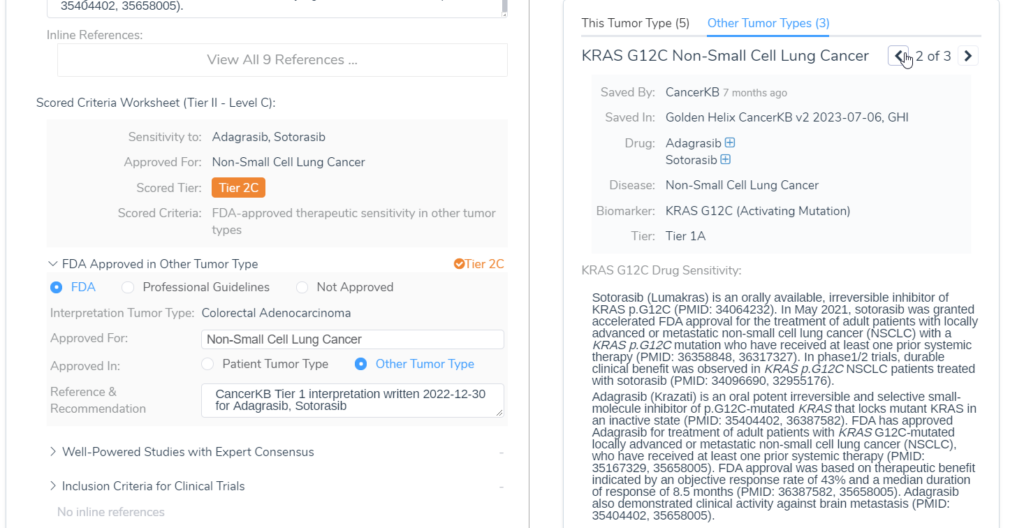
An example of this feature in action is KRAS G12C in Colorectal Adenocarcinoma. Sotorasib and Adagrasib are FDA-approved therapies for the KRAS G12C biomarker in non-small cell lung cancer, according to Golden Helix CancerKB. When this evaluation option is set, when the KRAS G12C variant is added for a patient with colorectal adenocarcinoma, a tier II C interpretation will automatically be included in the evaluation, letting you know that this could be a possible therapy for the patient.
Now that the box has been checked to add tier II C interpretations to our evaluation if therapies are approved in a tumor type outside of the patient tumor type let’s explore how evaluation scripts can be used to add tier II C interpretations for inclusion in clinical trials.
Once you create an evaluation with all of the patient’s variants, which should now include interpretations for approvals in other tumor types, you have two options:
- Option 1: Use the “Add Associated Clinical Trials” evaluation script, which adds clinical trials for the existing interpretations for the patient biomarkers. This script will add active clinical trials for the drugs associated with the interpretations (and biomarkers) within the evaluation and could then be included in a clinical report for the patient.
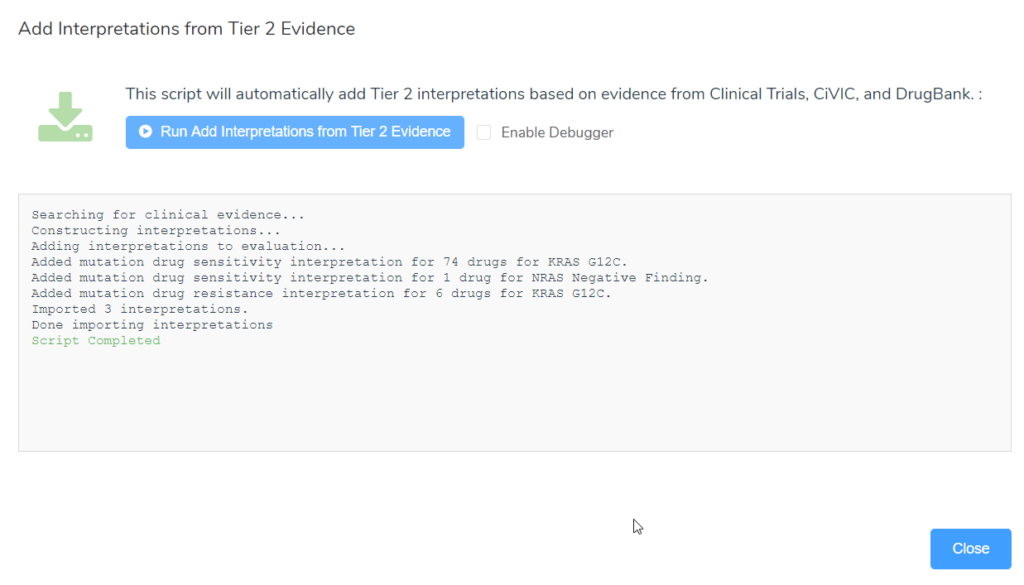
- Option 2: Use the “Add Interpretations from Tier 2 Evidence” evaluation script, which will actually create new tier II C interpretations for the evaluation based on evidence from clinical trials, CIViC and DrugBank.
In the Evaluation Scripts section of the evaluation, the little blue plus icon will show you all of the options for automating and customizing your patient evaluations (there is a lot of fun material here, and I recommend trying these all out!) Also, these evaluation scripts can be set up to automatically run with VSPipeline.
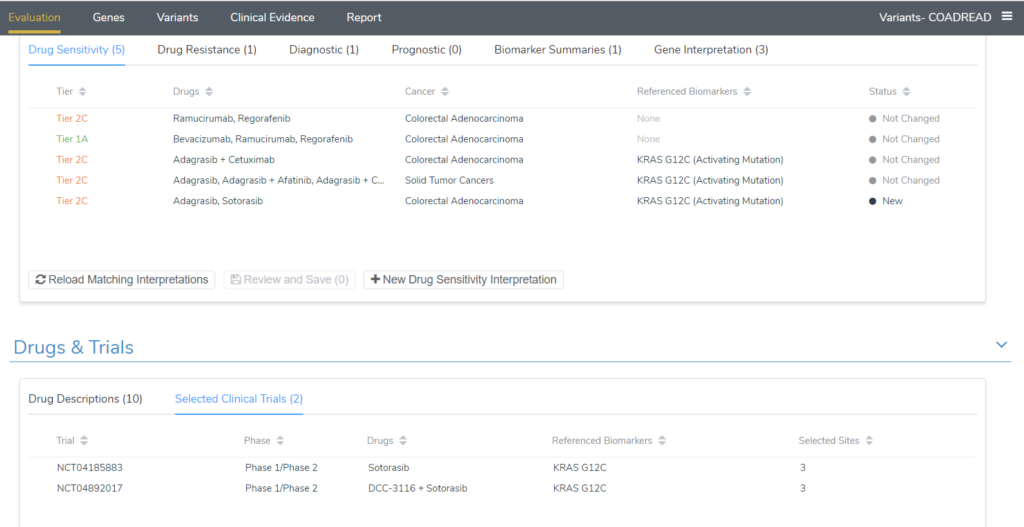
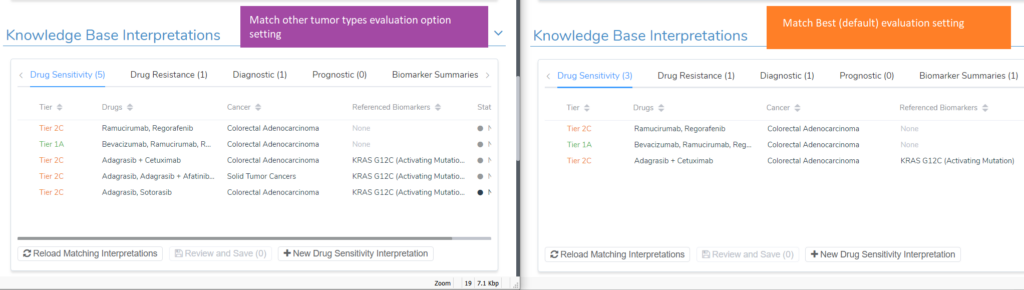
To see these scripts in action, I will continue to use the KRAS G12C example for a colorectal adenocarcinoma patient. Upon adding the KRAS G12C variant to the evaluation, 5 drug sensitivity interpretations were added; four tier II level C interpretations and one tier I level A. The image below shows the comparison of the evaluation option to match previous interpretations for all tumor types vs the default match best evaluation option. The additional tier II C interpretations that were included are one for drugs approved for Solid Tumors and a second interpretation that has Sotorasib and Adagrasib, which are approved for non-small cell lung cancer.
After running the “Add Associated Clinical Trials” script, 2 clinical trials were added to the evaluation for the drug Sotorasib. This means that the other drugs in the evaluation do not have any active trials for KRAS G12C colorectal adenocarcinoma patients.
Alternatively, after running the “Add Interpretations from Tier 2 Evidence” evaluation script, a number of new interpretations for not only drug sensitivity but also drug resistance that apply for the patient have been added. The image below is the output after running the script, summarizing the new interpretations that were added. Three interpretations were added, 2 tier II drug sensitivity interpretations (KRAS G12C and NRAS Negative finding) and one drug resistance interpretation for KRAS G12C.
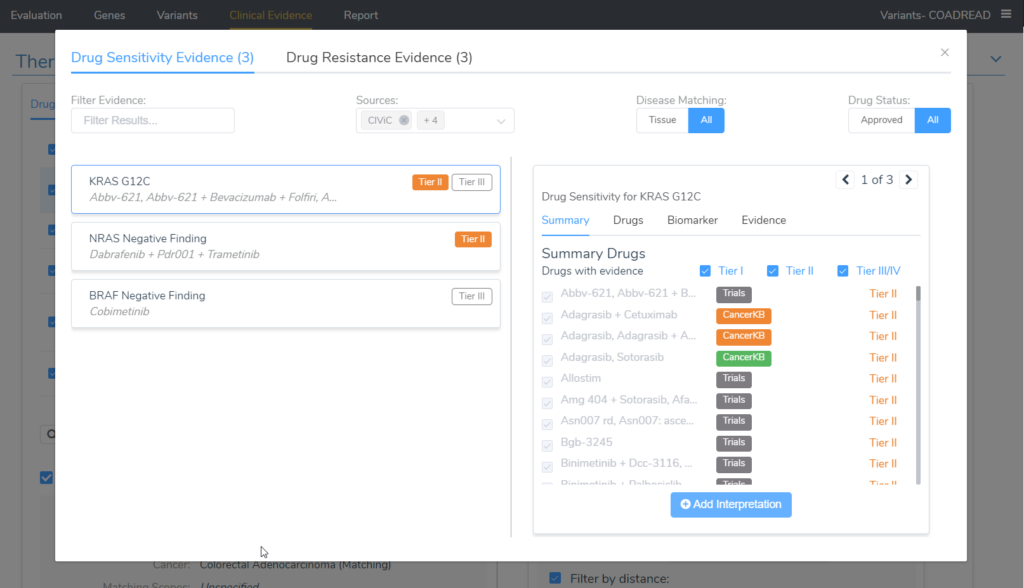
A nice way to look at all of the evidence for the variants in the evaluation is in the Clinical Evidence tab and selecting the Search All Drug Evidence button underneath the therapeutic options table. The image below displays nicely that all of the tier II evidence for the biomarkers in the project were added.
Looking at the image above, the tier II evidence is being shown for KRAS G12C and contains a very long list of drugs with the source providing the evidence (shown on the right side of the image). The second biomarker on the list that applied to this patient was an NRAS Negative finding, which has its own tier II evidence. Switching the selection at the top of this dialog to Drug Resistance Evidence would allow us to see all of the evidence for the tier II resistance interpretation.
Within the Clinical Evidence tab, we can also see the interpretations that were automatically created after compiling all of the tier II evidence for each biomarker, along with clinical trial information.
Capturing tier II information is always challenging as it 1) requires looking at drugs approved in other tumor types for the biomarker, i.e., searching through the NCCN Guidelines, FDA, EMA, etc. approvals, 2) searching through active clinical trials and 3) scouring cancer resources like DrugBank and CIViC for studies that provide some evidence about certain drug treatments, for the patient’s biomarkers and tumor type. So much time and effort! Using these 2 very special evaluation scripts consolidates all of that time and effort into a script that takes minutes, if not seconds, to run.
I hope you have been convinced to give this workflow a try! If you have questions or would like more guidance on how to implement the capture of tier II interpretations via evaluation scripts or VSClinical AMP in general, our support team is here to help! Just reach out to [email protected].