While the interpretation of germline variants generally focuses on the pathogenicity of a variant for a specific disease, the interpretation of somatic variants is centered around each variant’s impact on clinical care. As a result, clinical trials play an important role in assessing the clinical significance of somatic biomarkers, with the AMP Guidelines assigning a higher level of evidence to biomarkers that serve as inclusion criteria for clinical trials. Furthermore, clinical trials can provide additional treatment options to the oncologist, including treatments that are not yet approved or are approved for a different tumor type.
Until recently, VarSeq’s clinical trials annotation was based on the database of clinical trials provided by the National Cancer Institute (NCI). This database includes all trials which are sponsored or otherwise financially supported by the NCI. While this database is an excellent resource for cataloging clinical trials within the United States, it falls short when it comes to trials in other countries. Of the approximately 9,500 trials in the February release of NCI Trials, over 70% were exclusive to the United States. The restrictive nature of these trials severely limits the utility of this database for our many Asian and European customers.
Expanded Cancer-Related Clinical Trials
To improve our support for searching clinical trials around the world, we have begun incorporating all cancer-related clinical trials from ClinicalTrials.gov into our annotation track alongside trials from the NCI. This has more than doubled the number of trials in our database and has greatly expanded the number of Asian and European trials, with over 67% of trials having sites outside of the United States.
What can searching for clinical trials in VSClinical do for you?
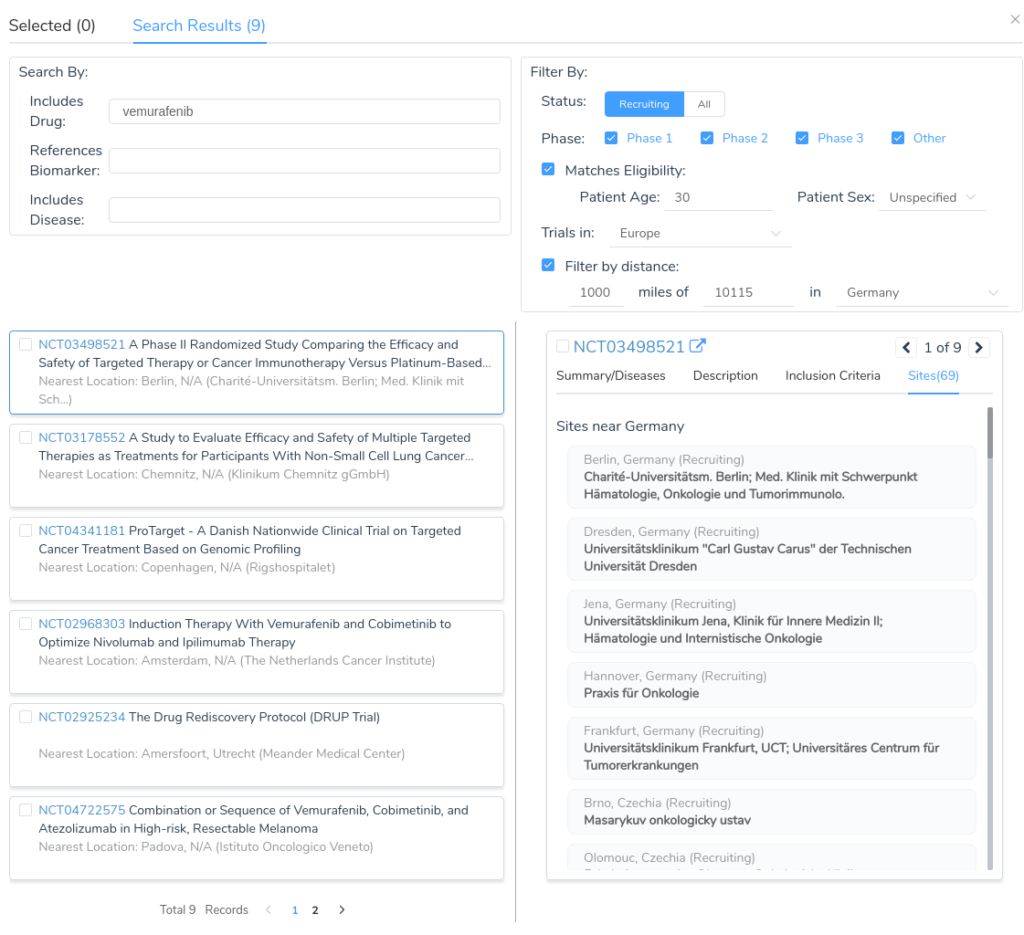
International Postal Code Queries
In addition to these improvements to our database, we have also been making significant improvements to our clinical trial search capability in VSClinical. Previously, postal code queries were only supported within the United States and trials in other countries could only be queried by selecting the specific country of interest. In the upcoming VarSeq release, postal code queries will be supported for most major countries. Distance filters will be applicable across countries by specifying a region of interest, such as Europe, Asia, or North America.
In addition to the improved country and region search, we have also added the ability to filter by phase and eligibility. The upcoming release will support filtering on various inclusion criteria, including:
- Patient age
- Patient sex
- Ease of the clinical trial
These updates significantly improve the clinical trial search capabilities in VSClinical and provide our users with the ability to explore clinical trials all over the world. We are looking forward to officially releasing these capabilities to our users! If you have any questions or comments about our improved clinical trial search capabilities or about our software in general, feel free to enter them into the comments below.
Why Search for Clinical Trials with VSClinical?
We’ve been trusted by doctors and scientists around the world to deliver reliable, accurate interpretations at scale. Our software is built from the ground up to be compatible with any existing lab and to deliver results with convenience, accuracy, and ease of use in mind. Whether you’re an existing VarSeq customer who’s still learning about our VSClinical add-on or you’ve found us on your search for a new variant interpretation service, take a look for yourself. Book a demo today!
Who knew searching multiple databases for clinical trials could be so easy?